Computerized Systems Validation (CSV)
The term computerized systems validation refers to a set of validation activities performed on the computerized systems of a pharmaceutical company that are critical from a GMP perspective. This scope is typically expanded to include GxP-relevant systems, meaning that validation of computerized systems should be conducted for all GxP-critical computerized systems. These systems may be relevant not only to GMP but also to GDP, GAMP, GCP, GLP, and other related codes of practice.
Validation of computerized systems encompasses both validation activities related to equipment/systems (i.e., qualification) and activities related to software, procedures, and other elements, which can collectively be referred to as processes (i.e., validation). Therefore, computerized systems validation incorporates aspects of both equipment qualification and process validation.
Computerized systems validation should not be seen as a one-time activity but as a set of established validation processes governing the entire lifecycle of a computerized system – from design and implementation to use and decommissioning – ensuring compliance with GxP requirements throughout its entire lifecycle.
In EU GMP Guidelines Annex 11: Computerised Systems, the following definition of lifecycle is provided as it applies to a computerized system:
“Life cycle: All phases in the life of the system from initial requirements until retirement including design, specification, programming, testing, installation, operation, and maintenance.”
Since validation activities for computerized systems should begin very early in their lifecycle, these activities must align with the entire pharmaceutical facility/system design process and are closely integrated with it.
Validation of computerized systems is an extensive and specialized set of multidisciplinary activities requiring the involvement of various competencies, including process technologists, quality assurance, IT, instrumentation/automation, and engineering services. All members of the validation team should be trained in the principles of computerized systems validation.
The most comprehensive guidelines describing the policies, methods, and tools for validating computerized systems, as well as ensuring that a system complies with GxP requirements throughout its lifecycle, are provided in the ISPE GAMP (International Society for Pharmaceutical Engineering / Good Automated Manufacturing Practice) series of guides, including GAMP5 and the Records and Data Integrity (RDI) guidelines. The ISPE Guidelines are not regulatory documents but are advisory in nature.
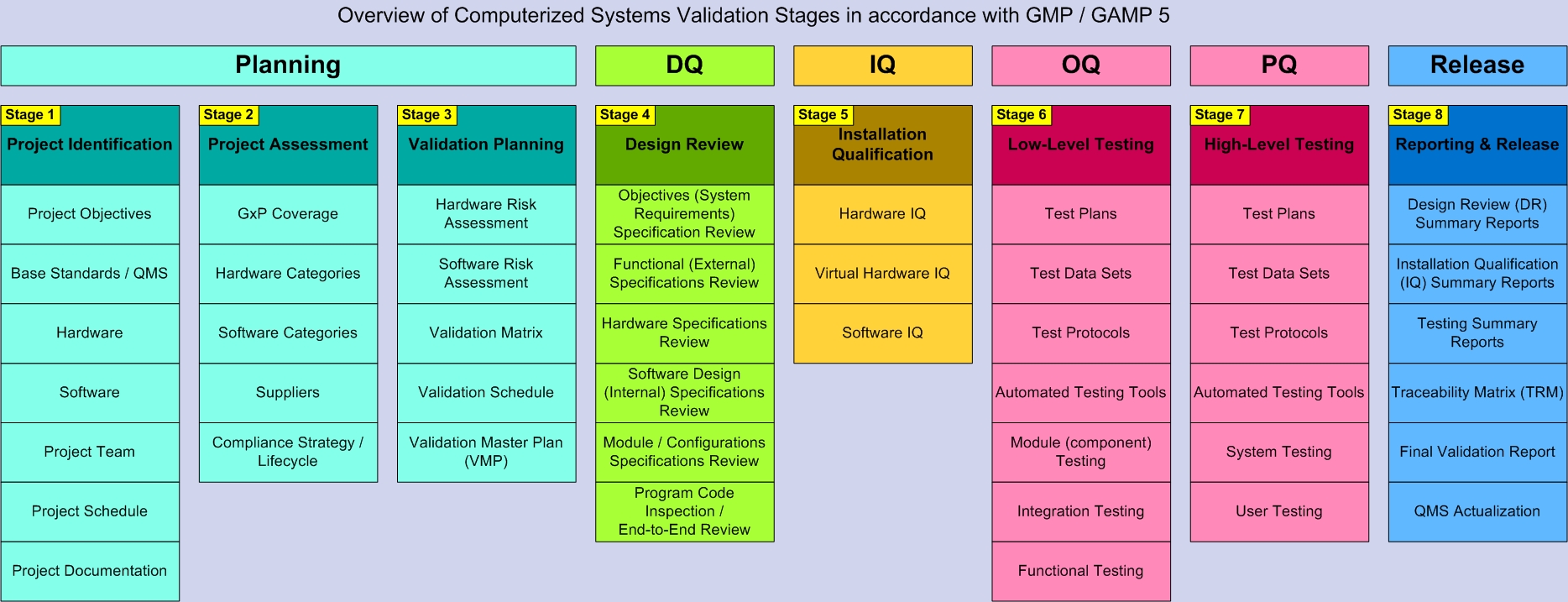
The validation of computerized systems can generally be divided into the following steps:
- Project Identification
- Project Evaluation
- Validation Planning
- Design Review (DR/DQ)
- Installation Qualification (IQ)
- Low-Level Testing (OQ)
- High-Level Testing (PQ)
- Reporting & Release
The activities/tools associated with each stage can be presented as follows:
The specialists at Tarqvara Pharma Technologies have years of experience in conducting qualification, validation, and acceptance tests within the pharmaceutical industry, in full compliance with international, European, and national GMP/GxP regulations and standards.
See also:
Qualification / Validation / Commissioning
Commissioning (FAT/SAT)
Risk-Oriented Approach
